HEPATITIS E
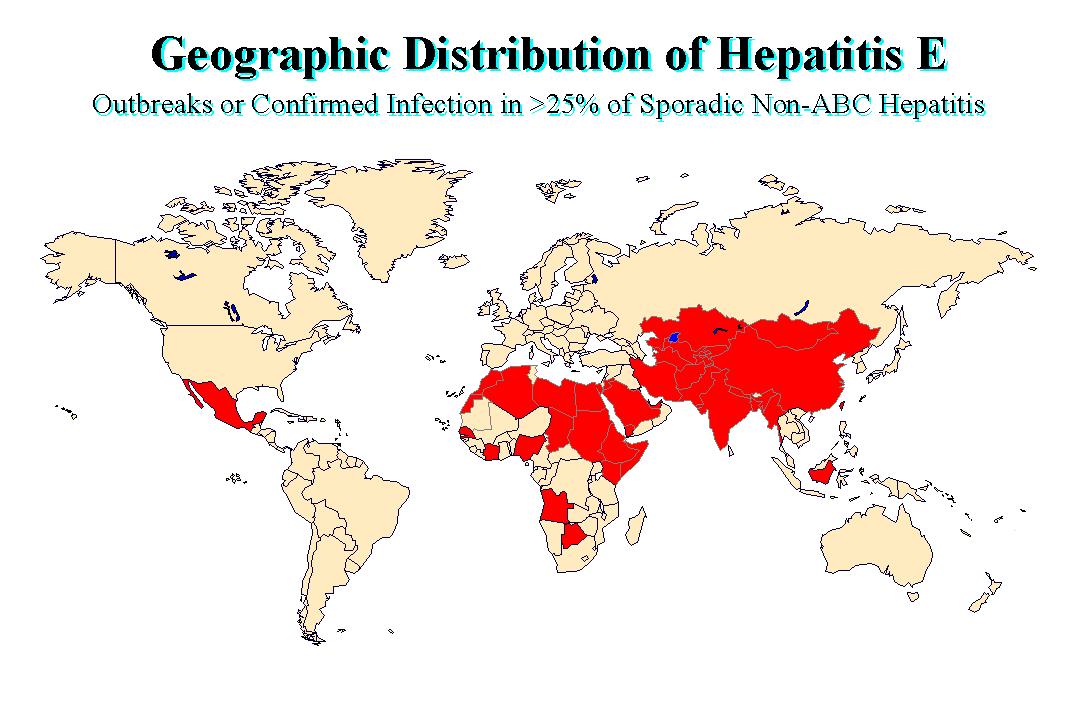
More than 50% of acute viral hepatitis occurring in some developing countries appears to be unrelated to infection by HAV or HBV and accumulating evidence suggests that a high proportion of this NANBH is enterically transmitted (Hepatitis E). Outbreaks of hepatitis E have been documented in the India, Pakistan, USSR, Borneo, Sudan, Ivory Coast, Algeria, and Mexico. These outbreaks affected young to middle aged adults and are often associated with a high mortality in pregnant women, approaching 20% in some epidemics. Several investigators described finding 27 to 34nm virus-like particles in the stools of acutely infected stools by IEM. Virus in the infected stools were transmitted to monkeys and chimpanzees and other primates who excreted similar 27 to 34nm in the stools in the pre-acute phase, and were shown to seroconvert. The disease had also been transmitted to a human volunteer using a pool of 14 stool extracts from infected patients. It is now classified as a member of the genus Orthohepevirus in the Hepeviridae family
PROPERTIES
Unenveloped RNA virus, 32-34nm in diameter
+ve stranded RNA genome, 7.6 kb in size.
4 genotypes: genotype 1 (Asia), genotype 2 (Africa and Mexico), genotype 3 (Europe and North America) and genotype 4 (Asia)
very labile and sensitive
Recently cultured
NATURAL_HISTORY
Hepatitis E disease occur in both sporadic and epidemic forms and is primarily associated with the ingestion of faecally contaminated drinking water. Little secondary household transmission has been documented to occur unlike HAV. Hepatitis E was first documented in New Delhi in 1955 when 29000 cases of icteric hepatitis occurred following the contamination of the city's drinking water. Several other large epidemics have occurred since in the Indian subcontinent and the USSR;
1. The Delhi Epidemic 1955-56
2. Ahmedaban, India 1975-76
3. Pune, India 1978
4. Kashmir 1980
5. Tashkent, USSR 1983
These outbreaks were originally thought to be due to HAV but retrospective analysis of stored serum identified HEV as the causative agent. Epidemics have also been reported from parts of China, Africa and Mexico. Sporadic cases predominantly occur in the Indian subcontinent. One notable feature of hepatitis E is the relatively low incidence of clinical disease in case contacts. For example, only 2.4% of household contacts of HE cases developed clinical illness during the 1981-82 epidemic in Kathmandu Valley, whereas the secondary attack rate for HAV was 10 to 20% among household contacts in the same region.
HEV has a slightly longer incubation period than HAV (2-9 weeks,
mean = 6 weeks) Like HAV, the disease is usually self-limiting.
The clinical attack rate is from 0.7 to 10%. Virus particles are
present in the faeces just before the onset of jaundice and the
appearance of antibodies. The particles are 27- 30 nm. There is a
good animal model available. Various strains of HEV had been
isolated in different primates. A particular strain may produce
disease in one type of animal but not another. Therefore,
different strains appears to have different host
susceptibilities. However, cross-protection can be demonstrated
between different strains. Therefore, the antigen responsible for
producing neutralizing antibodies appears to be the similar.
Clinically, the disease is similar to hepatitis A, but carried a higher mortality, especially amongst pregnant women. HAV has a mortality of 0.1 - 0.2%, in contrast with HEV which carries a mortality of 1-2%. In pregnant women, the case-fatality rate can be as high as 10-20%. The case fatality of HAV for pregnant women is the same as in the general population. The highest attack rate occurred among the 15 - 40 age group. Hepatitis E Accounts for 12-50% of clinical hepatitis in the Indian subcontinent. The epidemiology and clinical presentation of HEV infection vary greatly according to geographic location, which is based primarily of the prevalent genotypes. HEV genotypes 1 and 2 primarily infect humans, whereas genotype 3 and 4 mainly infect mammals with occasional cross over to humans.
Certain questions about the virus remains unanswered, Firstly, why should those aged between 15-40 should be predominantly affected. This implies that infection either occurs subclinically or not at all in younger individuals. This is supported by a study carried out in Kenya during an investigation of an HEV outbreak among refugees. Also why should the disease carry a higher mortality amongst pregnant women which is not the case with HAV and HBV. The unexpected high mortality rate in HE-infected pregnant women may reflect a unique feature of viral replication and pathogenesis of disease in these women. Indigenous immunoglobulin had been reported to be of use in the prophylaxis against hepatitis E.
Commercial assays are becoming available for the detection of
IgG using recombinant antigens and synthetic polypeptides. The
sensitivity and specificity of these assays are highly
questionable as they tend to give a high seropositive rate eg.
there is a seropositive rate of 2% in the US even though there had
not been any reported cases of hepatitis E in the US. The IgM
response is unreliable and is thought to only occur in 50% of
patients. RT-PCR assays are also available for the detection of the virus RNA.
There is no specific therapy for HEV infection, although ribavirin has been reported to be of some benefit in treating chronic infection, especially in transplant recipients. Other possible treatments include pegylated interferon or a combination of ribavirin and pegylated interferon.
Improvement in sanitation remains the most important means of
preventing HEV transmission. A recombinant vaccine called HEV 239 has been
developed and is licensed for use in China.
CDC
hepatitis branch; "Epidemiology and Prevention of Hepatitis
A-E: An Overview"