Parvoviruses
Parvoviruses are significant pathogens in the veterinary sciences. They are particularly associated with reproductive failure. The family Parvoviridae encompasses 3 genera which infect a variety of host species. They are small naked icosahedral viruses with a genome of a single-stranded DNA. The desonucleosis viruses infect insects. The dependoviruses requires a helper virus e.g. adenovirus or HSV for replication. They have been described in a number of mammalian species including man but have yet to be linked with any disease. The third genus consist of autonomously replicating parvoviruses which are frequently associated with disease. 3 serologically distinct human parvoviruses have been recognized:-
(1) human parvovirus B19
(2) parvovirus particles in stool specimens - the precise pathogenic role of these agents remain unclear, eventhough animal parvoviruses have been shown to cause diarrhoea in animals.
(3) more recently a parvovirus RA-1 was isolated from the synovial tissue of cases of RA. However, this is now thought to be a contaminating bovine parvovirus.
In B19, + stranded DNA and - stranded RNA are packaged in roughly equal numbers. When DNA is extracted from the capsid, the + and - strands come together. This is a feature of dependoviruses. B19 is an exception to the rule as other autonomously replicating parvoviruses have more than 90% of their particles with +DNA. The genome has 2 reading frames, 1 codes for a non-structural protein, the other code for the structural proteins, VP1 and VP2. VP2 make up to 90% of the mass of the virus. There is only 1 promoter present. Parvovirus replicate in nucleus of host cell, requires functions provided by
a. Host cell in S phase - genus Parvovirus
b. Helper virus - genus Dependovirus
The adeno-associated viruses are fairly common. the seroprevalence is high in the general population. This class of parvoviridae are able to cause true latent infection in the absence of helper virus. They can integrate at specific sites in the human genome e.g. at a site at chromosome 19. The integrated dependovirus genome can be rescued by infection with a helper virus.
Human Parvovirus B19 was discovered by Cossart et al. in 1975. During screening for HBsAg in blood donations, they found several specimens with a positive immunoelectrophoresis result but negative haemagglutination and RIA results. In 11 of these sera, One of which was encoded B19, parvovirus like particles were found in the sera on electron microscopy. The authors speculated that this agent could be a novel infectious agent. It was not until several years later that disease associations were found for B19. The most prominent of which is erythema infectiosum (Fifth disease), which had been recognized for many years as one of the classical rash illnesses of childhood.
A. Properties
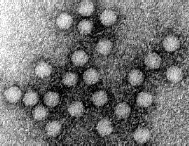
ss DNA naked icosahedral virus
32 capsomers with 3 proteins of 48K 68K and 80K
DNA 5.5 kb in length
Approximately equal proportions of DNA of +ve and
-ve polarity are found in separate particles
B19 virus is antigenically distinct from the viruses
found in faeces, RA-1 and other parvoviruses
Parvovirus particles. (Courtesy of Linda M. Stannard, University of Cape Town)
B19 is associated with the following:-
Volunteer studies where volunteers were inoculated intranasally with B19 (Anderson et al 1985) provided proof of B19 in human disease. The virus sets up a systemic infection with copious viraemia one week after inoculation. At the same time, virus is shed from the respiratory tract. As the viral titres fall IgM appears. There is a delay of several days before IgG appears. Reinfection was seen in 1 volunteer.
During viraemia, reticulocyte numbers fall to undetectable numbers, recovering 7 to 10 days later. There is a fall of 1g of Hb in normal people. Lymphopenia, neutropenia and thrombocytopenia also occur. The symptoms of erythema infectiosum occurs late in the course of events, with the rash appearing 17 - 18 days after inoculation, and arthralgia a day or so later. These symptoms are likely to be immune mediated. It appears that an early erythrocyte precursor is susceptible to B19 infection.
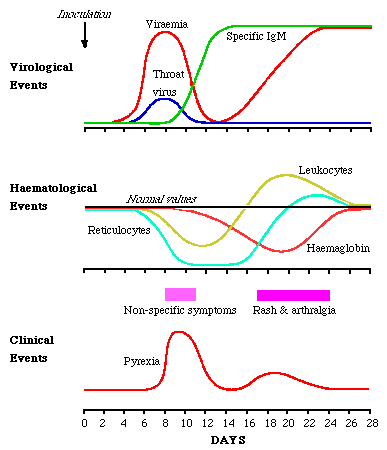
From Course BS335 Lecture Notes by Dr. Alan Cann
B19 virus is present throughout the year, in temperate
climates outbreaks of infection are more common in the spring and
summer. These outbreaks are centred around primary schools where
up to 40% of pupils may be infected. Infection is commonest
amongst 4 - 10 yr olds. By adulthood 60% of the population are
seropositive. Respiratory spread is the usual route of
transmission of the virus. Bloodborne spread can occur ( 1 in
40000 blood donations have virus present ) in recipients of whole
blood and factor VIII. The frequency of seropositivity among
haemophiliac children is significantly higher than normal.
The common manifestation of B19 infection is a mild febrile illness with a maculopapular rash. In children, a slapped cheek appearance is common. In adults, this is less common but the rash is frequently followed by joint involvement.
1. Erythema Infectiosum;- 7 days after inoculation, a prodrome consisting of fever, headache, chills, malaise and myalgia is common which accompanies the viraemic phase of infection. There is a period of 7 before the onset of the rash. The classical rash of Fifth disease occurs in 3 stages. Firstly the rash appears on both cheeks (slapped cheek appearance). The second stage appears 1 to 4 days afterwards with the appearance of a erythematous maculopapular rash on the trunk and limbs. The third stage of the rash is highly variable in duration, lasting from 1 to 3 weeks, and is characterized by marked changes in the intensity of the rash with periodic complete evanescence and recrudescence. The rash is often pruritic, and is more prominent on the extensor surfaces. Subclinical infection can occur especially in children. Reinfection has been documented. There is wide variation in the form of the rash, in many cases the rash is indistinguishable from rubella. The most common complication is joint involvement. Symptoms commence 1 to 6 days after the onset of the rash but may also occur in the absence of the rash.
Joint involvement is rare in children but occurs in 80% of adult females with a rash. In children, both sexes are equally affected and symptoms seem to be more severe than adults and of longer duration. In adults, females are much more likely to be affected. The most common presentation is arthritis affecting the small joints of the hand, followed by wrists, ankles, knees and elbows. Shoulders, cervical and lumbar spine as well as the hips may be involved. There is pain and stiffness in the joints which may be accompanied by minor swelling. Two thirds of the cases resolve by 2 weeks and the majority by 4 weeks.
2.Aplastic Crisis;- The aplastic crisis which occurs in patients with chronic haemolytic anaemia (such as sickle-cell disease, hereditary spherocytosis, B-thalesessaemia, pyruvate kinase deficiency) has been recognized for several decades. Typically, the patient has a viral-like illness with fever and constitutional symptoms, followed by the onset of fatigue and anaemia. The Hb level may fall as low as 4g and reticulocytes are undetectable, but the leucocytes and platelet counts usually remain normal. Bone marrow aspiration shows a marked reduction in the numbers of erythrocytic precursors present with other cell lines being normal. A transfusion of packed cells may be necessary to correct the severe anaemia. However the patient usually quickly recovers within a week, with the Hb recovering to normal levels. Such crises generally occur in children, tend to cluster both in time and location and do not usually recur in an affected individual. It was suggested as early as 1960 that aplastic crises may be caused by an infectious agent but it was not until the early eighties that B19 became associated with aplastic crises. In individuals with chronic haemolytic anaemias, B19 infection causes a profound reticulocytopenia may result in the depression of Hb levels to critical levels. Reports of erythematous rash after aplastic crisis are rare. B19 infection does not invariably result in aplastic crisis in patients with chronic haemolytic anaemias. Although it may be responsible for up to 90% of aplastic crisis.
B19 infection in the immunocompromized;- A B19 associated illness characterized by a persistent or a remitting and relapsing anaemia occurs in immunocompromized individuals. HNIG may be useful in these patients as it will decrease the viral titres.
3. Infection in Pregnancy;- Infection by parvovirus during pregnancy is not associated with increased risk of fetal malformation. However infection during pregnancy is associated with increased fetal loss. This may be due to the fact that parvovirus attacks reticulocytes which may lead to anaemia in the fetus and death. Indeed fetal hydrops (which is recognized as the most severe manifestation of rhesus incompatibility disease, with IUD, anaemic heart failure, pleural effusion, hepatoslenomegaly and ascites) is a consistent feature in still born infants. Infection in the first trimester is associated with a 5 - 10% fetal loss. Infection in the second trimester 12.5%. Clearly the majority of pregnancies proceed to term with delivery of normal infants. However fetal hydrops as a result of second and third trimester infection is seen in the newborn. Maternal infection occurs 2 to 12 weeks prior to the diagnosis of fetal hydrops. A diagnosis of fetal hydrops may be made by ultrasound.
In a PHLS prospective study in pregnancy. 190 women known to be infected with B19 during pregnancy were followed up. A satisfactory outcome in 84% of women. No congenital abnormalities were observed. There was substantial risk of fetal loss - especially in the 2nd trimester. The transplacental transmission rate was 33%. The fetal death rate was 9%. B19 appears to be responsible for a 20 fold increase in fetal loss during the 2nd trimester of pregnancy. B19 does not cause recurrent abortions.
4. Persistent infection in immunocompromized patients - B19 can cause persistent infection resulting in severe anaemia in the immunocompromised, particularly in children having immunosuppressive therapy. Patients with congenital immunodeficiency and AIDS may also develop this syndrome. Patients with this syndrome who have been given HNIG often show a beneficial response. However, improvement is usually seen when immunosuppressive therapy is relaxed.
The differential diagnosis of erythema infectiosum includes all diseases where a maculopapular rash may be present eg. rubella, enteroviruses, arboviruses, streptococcal infection, allergy. Rubella causes the greatest problems as the two viruses may circulate together. Therefore a definitive diagnosis can only be made by serological tests. Aplastic crisis in a patient may be diagnosed by finding a reduction in Hb of >= 2g and a reticulocyte count of less than 0.2%. Although B19 infection is the commonest cause of aplastic crisis, bacterial infections ( eg. pneumococcal septicaemia ) or marrow suppressive drugs such as chloramphenicol can cause the same condition.
Detection of Virus;- The diagnosis of aplastic crisis may be made early in the disease by the detection of the virus. This may be accomplished simply and rapidly by countercurrent immunoelectrophoresis of the patient's serum against parvovirus Ab. A +ve result can be confirmed by direct or immune EM. The virus will be detected in 30% of the cases where a specimen is taken within 24 hrs of the onset of symptoms. A more sensitive method such as RIA or DNA-DNA hybridization, or PCR may increase this detection rate to more than 60%. The diagnosis of B19 in a fetus depends on the detection of the virus. As maternal infection will have occurred some weeks earlier and both maternal and fetal sera will probably be B19 IgM -ve. ( In contrast to rubella where the infection would have occurred months earlier so that maternal IgM is no longer present and so that the presence of IgM in the fetus would be diagnostic of congenital infection ). However there is often a persistent viraemia in the fetus. Thus the diagnosis can be made by the detection of the virus in fetal blood samples or autopsy material where DNA-DNA hybridization and PCR can be carried out.
Antibody Detection;- Counter-immunoelectrophoresis
using B19 virus as antigen, detects antibody of all classes.
Class specific Ab may be detected by ELISA or RIA. Current tests
employ the principle of "antibody capture" whereby the
class specific patient antibody is bound to a solid phase coated
with antibody to IgM or IgG. Diagnosis of B19 infection can be
made by the detection of either (i) B19-specific IgM or (ii) a
rising titre of B19 specific IgG. B19 specific IgM may be
detected in such tests up to 3 months after the onset of
symptoms. B19 IgG lasts longer but this antibody is not
detectable lifelong following infection. therefore caution must
be exercised in interpreting the results of such tests in a
screening situation ; patients with no detectable IgG may
nevertheless have experienced previous B19 infection.
The only specific treatment for B19 infection is the administration of HNIG in cases of persistent B19 infection in the immunocompromised. Controlled trials have not been done but these cases are rare and HNIG is worth giving where there is persistent viraemia. Symptomatic therapy for erythema infectiosum is rarely necessary. Cases of aplastic crisis require transfusion of erythocytes until a satisfactory Hb level is obtained. Consideration should be made to giving HNIG to susceptible patients with chronic haemolytic anaemias who requires short-term protection e.g. if they are in the same ward as a patient having a aplastic crisis. In any case they should be isolated from patients with B19 induced aplastic crisis.
If B19 infection occurs during pregnancy, there is no cause for alarm. The pregnancy should be allowed to proceed and carefully monitored. At delivery examination of the cord blood for B19 IgM will reveal whether the virus has crossed the placenta and infected the virus. The child should be carefully followed up for several to look out for any delayed sequelae.